
Time to a Solution
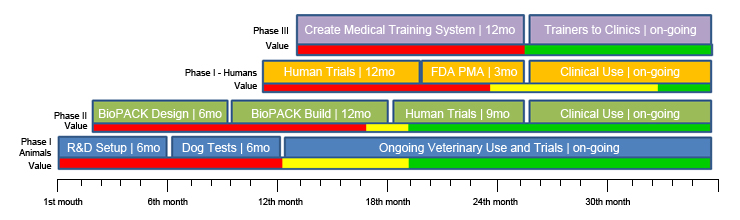
Because Reveal Therapies' patented BioPACK™ is a family of medical devices, FDA approval takes one fifth (1/5th) of the time, and costs one-fiftieth (1/50th) as much, as FDA approval for a medical drug. Therefore, patients could be treated with the BioPACK™ in as soon as 18 months after initial funding. Initial results and a product can be achieved in the veterinary arena within a year. Movement to human markets will be achievable within 18 months with almost immediate availability on an individual patient basis; and it is expected that company will be able to accomplish overall revenue independence by late in the 2nd year of operations. This is five times faster (5x) than can be done in any kind of drug-based care delivery.
Reveal's plan of action for moving the "TRAC-EM™" from the lab, is to go into full animal trials, in dogs, under FDA IDE safety rules; and by extension, opening the technology up to pets, within six months. This will allow us to build better data and establish a full body of evidence for predictable, repeatable: reliability and scalability; very quickly. The second round of actions will take the device through the full FDA IDE process to market approval, as well as an international CE mark, and develop the BioPACK™ for miniaturization using microfluidics within 15-18 months. The third round of actions will open-up the device into direct human treatment and establish its presence in centers of excellence, expand the synergistic medical educational mechanism, and through the completion of microfluidic miniaturization, radically expand its presence and cost effectiveness for both humans and pets.
Reveal's plan of action for moving the "TRAC-EM™" from the lab, is to go into full animal trials, in dogs, under FDA IDE safety rules; and by extension, opening the technology up to pets, within six months. This will allow us to build better data and establish a full body of evidence for predictable, repeatable: reliability and scalability; very quickly. The second round of actions will take the device through the full FDA IDE process to market approval, as well as an international CE mark, and develop the BioPACK™ for miniaturization using microfluidics within 15-18 months. The third round of actions will open-up the device into direct human treatment and establish its presence in centers of excellence, expand the synergistic medical educational mechanism, and through the completion of microfluidic miniaturization, radically expand its presence and cost effectiveness for both humans and pets.